
BOSTON NEURODYNAMICS CERTIFIED NEUROTHERAPIST CLASS OF 2022
THINGS TO KNOW BEFORE YOU CHOOSE WHERE TO GO RECEIVE YOUR NEUROFEEDBACK ?
Overview of the issue
Neurofeedback equipment registered with the FDA is considered a medical device. There are companies tha follow these guidelines. A medical device is supposed to be sold only sold to licensed providers. There are a few companies selling neurofeedback instruments who will sell to anyone, not just licensed providers. Some of them have not registered their devices as medical devices with the FDA. That’s because the FDA guidelines, until recently, have seemed to be somewhat ambiguous regarding neurofeedback equipment, nor has the FDA paid much attention to it.
A few companies who are registered with the FDA sell equipment to dealers. Some of those dealers sell the equipment directly to consumers with no licensed provider involved. It’s unclear if this is allowed under FDA guidelines. Many in the industry think it’s not allowed, but this too must be sorted out.
More detail on what are the FDA guidelines and what they mean
There are a number of equipment and software vendors for neurofeedback. Several of the top-tier companies are registered with the FDA and have gotten 510k certification from the FDA. Those documents are for the “intent to market a device.” This means they meet certain standards of manufacturing and quality-control based on strict FDA guidelines. The FDA does not assess the equipment, the software, or its quality as part of its 510k approval. It just ensures the company meets the process guidelines for manufacturing and quality.
There are other manufacturers of equipment and software who are not registered at all with the FDA. Information obtained recently from an FDA source suggests that all manufacturers of neurofeedback equipment and software should have submitted documents registering with the FDA. If that’s true, they are in violation of FDA guidelines. These companies could be subject to FDA scrutiny. There has been some uncertainty regarding FDA guidelines in the past. Some companies chose to interpret the guidelines in the way most conducive way to their goals without direct contact with the FDA.
What is biofeedback allowed to do under FDA regulations?
The FDA has “grandfathered” all biofeedback equipment as a device to promote relaxation. No other claims can be made. In 1976 when the FDA Medical Device Act was approved, relaxation was all they included under biofeedback at the time. Any company that submits and gets approval for FDA’s 510K is stating their equipment meets “substantial equivalence” to all previous equipment built in 1976 or before. That is, it can promote relaxation. Once approved for that, no company has to go through the expensive process of being approved by the FDA to help with a condition such as ADHD or anxiety.
The equipment is being used widely to deal with all kinds of conditions. No manufacturer states any conditions that their equipment treats. They stay away from any such claims as any such claims would be instantly looked at harshly by the FDA.
Off-Label Use
Clinicians are a different story than manufacturers. They can use devices and medications “off-label” for patients as they see fit. No clinician should make any claims that neurofeedback cures ADHD or anxiety either. If they did, they’d have a problem with their licensing board. But they can use a variety of tools, including neurofeedback, to help in the process. Since neurofeedback primarily helps both relaxation and improvements in self-regulation, there is really little need to make claims for the technology. Clinicans are not subject to FDA regulation for use in these ways.
Will the FDA approve neurofeedback for treatment of ADHD, anxiety, and other conditions?
The cost to obtain approval for a device to treat ADHD or anxiety from the FDA is huge – millions and millions of dollars. No company will fund these efforts. Why? Any company who funds approval for neurofeedback to treat ADHD would also instantly get approval for all devices that currently exist since they are all considered “substantially equivalent” by the FDA. The company would open the market for their competitors. No one will put up funding under those conditions.
If not for that, many conditions would be able to get approval by the FDA. Certainly ADD/ADHD, anxiety, and depression would quickly gain acceptance after clinical trials. Many other conditions would also. Funding of the expensive clinical trials that are required by the FDA require huge investments no one is willing to make.
Who can equipment and software be sold to?
As a medical device, manufacturers of neurofeedback equipment with 510K FDA registration can sell only to licensed health providers. There may be exceptions in the few places that don’t license mental health providers. Even then, appropriate credentials are required.
No manufacturer can sell equipment to a consumer. A licensed health clinician can provide equipment to a client or patient. However, the responsibility for individual use would then fall under their licensure.
The problem with manufacturers of equipment that have not registered with the FDA is that they can sell directly to non-licensed providers without any recourse. As mentioned earlier, it appears that these manufacturers may be in violation of FDA guidelines, and that they too should not be selling directly to unlicensed parties directly, particularly consumers. However, the FDA guidelines were unclear until recently, and those guidelines are being more clarified.
There are manufacturers with 510K approval who have been selling directly to consumers and non-licensed individuals, or have been selling equipment to distributors who resell to anyone. This is also being looked at closely within the neurofeedback industry. The main professional organizations in the field, ISNR, AAPB, and BCIA are all concerned about consumers purchasing neurofeedback equipment without appropriate supervision or training.
Learn More
There is an ongoing effort within the field of neurofeedback to clarify the FDA requirements for EEG biofeedback equipment and software. We include the information that is currently believed to be correct and will continue to update this section with any clarifications we receive.
Brain training
What is Neurofeedback known to Improve?
Neurofeedback improves your brain’s ability to balance itself which results in a reduction or complete removal of your symptoms. A nervous system can become dysregulated in many ways and for a variety of reasons. This includes trauma you may have experienced in childhood through to adulthood including divorce, accidents, or illness. Symptoms often arise because the nervous system is in a state of defence against perceived threats in the world, which can often be outwith our conscious control.
No New Tricks, No old dogs.

Neurofeedback uses highly developed software to help your brain become aware of its own activity. This is represented in a feedback animation, game or even YouTube video you watch on a large TV. Its a bit like looking in a mirror to adjust your appearance – your brain will respond and adjust in a positive way when it sees its own activity on the screen. Although you’re not consciously aware of this process, you may notice reduction in symptoms from even the first session.
No Direct Stimulation
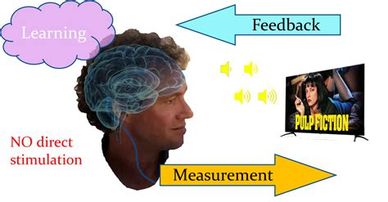
Neurofeedback Therapy is non-invasive and no electrical current is being sent to your brain. Sensors are attached to your head which pick up activity in your brain in the same way that a medical doctor listens to your heart from the surface of your skin. This ‘message’ is sent via an amp to a computer and then onto the screen. There is a visual and/or auditory feedback with whatever animation you choose to watch on the screen. Neurofeedback makes use of neuroplasticity and your brain’s innate desire for balance, returning it to a place in which it can cope much better with the world.
REMEMBER BASIC TRAINING?

Initially when training begins, the changes are short-lived but gradually the brain learns to stay in its healthier patterns. The process is similar to going to the gym to train muscles, we find your optimum training level and you repeat the training until stronger/healthier. Thankfully, unlike muscles, the brain learns to stay healthier, usually requiring around 20 sessions.
Science & History

If you’re interested in reading some of the science and history behind neurofeedback, PubMed has some accessible literature and please also keep an eye on our blog
THE PAST IN REVIEW

Now, just look at how Good you Feel, Now!
what is neurofeedback?

Clarification of Neurofeedback
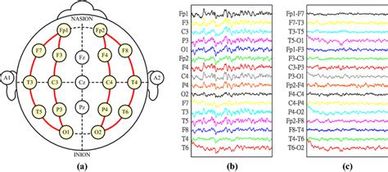
Like other forms of biofeedback, NFT uses monitoring devices to provide moment-to-moment information to an individual on the state of their physiological functioning. The characteristic that distinguishes NFT from other biofeedback is a focus on the central nervous system and the brain. Neurofeedback training (NFT) has its foundations in basic and applied neuroscience as well as data-based clinical practice. It takes into account behavioral, cognitive, and subjective aspects as well as brain activity.
NFT is preceded by an objective assessment of brain activity and psychological status. During training, sensors are placed on the scalp and then connected to sensitive electronics and computer software that detect, amplify, and record specific brain activity. Resulting information is fed back to the trainee virtually instantaneously with the conceptual understanding that changes in the feedback signal indicate whether or not the trainee’s brain activity is within the designated range. Based on this feedback, various principles of learning, and practitioner guidance, changes in brain patterns occur and are associated with positive changes in physical, emotional, and cognitive states. Often the trainee is not consciously aware of the mechanisms by which such changes are accomplished although people routinely acquire a “felt sense” of these positive changes and often are able to access these states outside the feedback session.
NFT does not involve either surgery or medication and is neither painful nor embarassing. When provided by a licensed professional with appropriate training, generally trainees do not experience negative side-effects. Typically trainees find NFT to be an interesting experience. Neurofeedback operates at a brain functional level and transcends the need to classify using existing diagnostic categories. It modulates the brain activity at the level of the neuronal dynamics of excitation and inhibition which underly the characteristic effects that are reported.
Research demonstrates that neurofeedback is an effective intervention for ADHD and Epilepsy. Ongoing research is investigating the effectiveness of neurofeedback for other disorders such as Autism, headaches, insomnia, anxiety, substance abuse, TBI and other pain disorders, and is promising.
Being a self-regulation method, NFT differs from other accepted research-consistent neuro-modulatory approaches such as audio-visual entrainment (AVE) and repetitive transcranial magnetic stimulation (rTMS) that provoke an automatic brain response by presenting a specific signal. Nor is NFT based on deliberate changes in breathing patterns such as respiratory sinus arrhythmia (RSA) that can result in changes in brain waves. At a neuronal level, NFT teaches the brain to modulate excitatory and inhibitory patterns of specific neuronal assemblies and pathways based upon the details of the sensor placement and the feedback algorithms used thereby increasing flexibility and self-regulation of relaxation and activation patterns.
NEUROFEEDBACK (NFL) OR EEG BIOFEEDBACK HAS..
Neurofeedback (NF), or EEG biofeedback, has been practiced for well over four decades. Hundreds of thousands of individuals and families impacted by various mental health and/or neurological conditions have benefited greatly from this powerful, effective, established, and proven intervention. NF is relatively non-invasive and creates lasting results in stark contrast from the outcomes derived from pharmaceutical treatment for a wide variety of conditions. We estimate over 15,000 clinicians, world-wide are using this technology. The represented professions are inclusive of: psychology, counseling, social work, marriage and family therapy, nursing, neurology, pediatrics, rehabilitation medicine, physical therapy, occupational therapy, naturopathic medicine, speech and language pathology, chiropractic, psychiatry, child and adolescent psychiatry, and family medicine.
MANY PRACTIONERS OF NF
Many practitioners of NF are certified through the Biofeedback Certification International Alliance (BCIA; bcia.org) which certifies individuals who meet specific education and training standards. NF certification requires a minimum of 36 hours of didactic training, passing a written exam, 25 hours of mentoring, 10 case reviews, performing 100 hours of client sessions, and 10 hours of personal NF. Further, BCIA certificants attest that they are either licensed clinicians or working under the supervision of a licensed clinician.
Contrary to claims that NF is insufficiently supported by research, here are links for you to review
Arns, M., de Ridder, S., Strehl, U., Breteler, M., & Coenen, A. (2009). Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clinical EEG and Neuroscience, 40(3), 180-189. doi:10.1177/155005940904000311 https://www.ncbi.nlm.nih.gov/pubmed/19715181
Coben, R., Wright, E. K., Decker, S. L., & Morgan, T. (2015). The impact of coherence neurofeedback on reading delays in learning disabled children: A Randomized controlled study. NeuroRegulation, 2(4), 168-178. doi:10.15540/nr.2.4.168 www.neuroregulation.org/article/view/15893/10087
Micoulaud-Franchi, J-A., Geoffroy, P. A., Fond, G., Lopez, R., Bioulac, S., Philip, P. (2014). EEG neurofeedback treatments in children with ADHD: An update meta-analysis of randomized controlled trials. Frontiers in Human Neuroscience, 8(906), 1-7. doi:10.3389/fnhum.2014.00906 https://www.ncbi.nlm.nih.gov/pubmed/25431555
Steiner, N. J., Frenette, E. C., Rene K. M., Brennan, R. T., & Perrin, E. C. (2014). In-school neurofeedback training for ADHD: Sustained improvements from a randomized control trial. Pediatrics, 133(3), 483-492. doi: 10.1542/peds.2013-2059. doi:10.1542/peds.2013-2059 https://www.ncbi.nlm.nih.gov/pubmed/24534402
Wigton, N. L., & Krigbaum, G. (2015). Attention, executive function, behavior, and electrocortical function, significantly improved with 19-channel z-score neurofeedback in a clinical setting: A pilot study. Journal of Attention Disorders, [e-pub ahead of print]. doi:10.1177/1087054715577135 https://www.ncbi.nlm.nih.gov/pubmed/25823743
MOREOVER
Moreover, as can be found in the ISNR Comprehensive Bibliography (www.isnr.org/resources), ISNR’s official peer-reviewed scientific journal NeuroRegulation (www.neuroregulation.org), and the archives of ISNR’s Journal of Neurotherapy (www.isnr-jnt.org), the research literature is substantial. For example, 1,447 peer reviewed journal articles are cited in the National Library of Medicine when using the search terms: ‘EEG Biofeedback’, and in recent years there has been exponential growth in publications related to neurofeedback.
This literature documents the efficacy of NF for numerous conditions, inclusive of: ADHD, mood disorders, anxiety disorders, obsessive compulsive disorder, epilepsy, substance use disorders, PTSD, autism, learning disorders, brain injury, insomnia, and headaches. While there are a few randomized trials showing that NF is equivalent to a placebo, there are a larger number that demonstrate greater efficacy.
Moreover, the number of randomized controlled trials showing efficacy are being published with growing frequency. For example, this study by Bessel van der Kolk, M.D. and colleagues was recently published in December 2016: van der Kolk, B. A., Hodgdon, H., Gapen, M., Musicaro, R., Suvak, M. K., Hamlin, E., & Spinazzola, J. (2016) A Randomized Controlled Study of Neurofeedback for Chronic PTSD. PLoS ONE 11(12): e0166752. doi:10.1371/journal.pone.0166752 https://www.ncbi.nlm.nih.gov/pubmed/27992435
Much of the research indicates that NF is frequently used as an adjunct to traditional treatments such as stimulants for ADHD, and it’s rare that NF is the only intervention. However, research and clinical evidence shows repeatedly that when NF is added to other therapies the outcomes are typically superior. Further, there are established CPT codes for insurance reimbursement of biofeedback, as NF is a type of biofeedback, which have been in place for close to 40 years; for which many insurers do reimburse.
Inquire with your therapist to learn if and which insurances they accept.
NEUROFEEDBACK and MEDITATION
Check out this great video
Mind Melds and Brain Beams: The Dawn of Brain-to-Brain Commu
Music students download the technique of their favorite pianist or singer directly into their brains.
Medical students download the skills of a seasoned surgeon or diagnostician. And each one of us routinely uploads our thoughts and memories to the digital cloud.
Prime Movers Lab
While these scenarios still lie in the future, rudimentary versions of the necessary brain-to-brain technology exist today. But the ability to directly influence another person’s brain raises serious questions about human rights and individual freedoms.
Black rock Neurotech - Move Again Brain Computer
This program is part of the Big Ideas Series, made possible with support from the John Templeton Foundation. Our media partner for this program is Popular Science. Footage of the brain-to-brain interface experiment provided by the University of Washington. For more information on the research:http://www.washington.edu/news/201
Meditation and the Brain | Dr. Stixrud & Dr. Travis |
A whole new meaning to Destin Florida’s favorite phrase: Look at Those Waves!
You can Ask Melanie Cassulo, LMHC about TM
See yourself, 3 weeks from today… what are you doing?
In your vision, is it before or after your visit to
Coastal Counseling Destin
Brainwaves on TM
You can Transcend Today
Mind, Music & Brainwaves
Why music makes us shiver
Coastal Counseling Destin
Music Recorded from the brain of a girl affected with cerebral palsy
What tune is your brain singing?
Exploring the Impact of Music on Brain Function
For more information go to http://nccam.nih.gov/news/events/IMle...
- Home
- DEPRESSION / ANXIETY
- PTSD / TRAUMA
- ADDICTION
- LIFESTYLES
- TRAINING & CERTIFICATIONS
- HYPNOSIS REFERRAL FORM
- HYPNOSIS
- FEARS Vs. PHOBIAS
- INSOMNIA
- WEIGHTLOSS
- SMOKING CESSATION
- NLP NEUROLINGUISTIC PRO
- HEALING SOUND FREQUENCIES
- HYPNOTIC REGRESSION
- BREATHWORK
- HYPNODONTICS
- LANGUAGE STUDY HYPNOSIS
- WORKSHOPS
- DOWNLOAD HYPNOSIS AUDIO
- NEUROFEEDBACK
- What is Biofeedback
- Contact Us Now
- Gottman Success Stories
- TELEHEALTH
- PROUD AMERICAN PROGRAM
- MERCHANDISE
- Appointment Booking
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.
